13. Respiration and Energy Transfer -
Part 01 - Formation of ATP
Formation of ATP :
Oxidative phosphorylation :
Anaerobic respiration :
Glycolysis :
do you know ?
1. Glycolysis
2. Some plant tissues which are modified to store starch (like potato) mainly depend upon glycolysis for energy production.
3. myoglobin of skeletal muscles is oxygen storing and transporting pigment .
Aerobic Respiration :
Conversion of pyruvic acid to Acetyl CoA :
Do you know?
Krebs Cycle ( TCA cycle/ Citric Acid Cycle):
Amphibolic Pathway :
Electron Transport chain (Electron transfer system) :
Significance of ETS :
always remember :
- Formation of ATP is called as phosphorylation.
- In nature, phosphorylation occurs in three different ways as -
- photophosphorylation
- substratelevel phosphorylation and
- oxidative phosphorylation.
- Formation of ATP in the chloroplasts in presence of light is called photophosphorylation.
- It takes place in the two forms.
- Cyclic photophosphorylation
- Non-Cyclic photophosphorylation
- It is a direct phosphorylation of ADP by transfer of a phosphate group from any suitable substrate.
- It occurs in cytoplasm of the cells and matrix of mitochondria.
Oxidative phosphorylation :
- It is phosphorylation of ADP at the cost of energy released during oxidation of substrates like NADH+H+ and FADH2.
- This occurs on the inner mitochondrial membrane only.
- When energy is required for any metabolic process, ATP is hydrolysed.
- ATP hydrolysis releases the energy which is used for the metabolic activities.
- Respiration is a catabolic process where in complex organic substrate is oxidized to simple components to generate biological energy.
- Cellular respiration occurs in two different ways -
- Anaerobic respiration
- Aerobic respiration.
Part 02 - Anaerobic respiration
Anaerobic respiration :
- Anaerobic respiration is the cellular respiration that does not involve the oxygen at all. It is also called as fermentation.
- It is completed through steps like
- glycolysis and
- conversion of glycolytic product to any suitable product like lactic acid, ethanol, etc.
Glycolysis :
- Glycolysis involves the breakdown of glucose molecule into two pyruvic acid molecules. Hence known as glycolysis.
- This is a common step in anaerobic as well as aerobic respiration.
- It occurs in cytoplasm of cell. It is completed in two phases as preparatory phase and pay-off phase.
- Overall process of glycolysis is completed through ten steps.
- First five steps constitute the preparatory phase through which glucose is phosphorylated twice at the cost of two ATP molecules and a molecule of fructose 1, 6-bisphosphate is formed.
- This molecule is split to form a molecule of glyceraldehyde 3-phosphate and a molecule of dihydroxyacetone phosphate.
- Both of these molecules are 3-carbon carbohydrates (trioses) and are isomers of each other.
- Dihydroxy acetone phosphate is isomerised to second molecule of glyceraldehyde-3-phosphate.
- Thus, two molecules of glyceraldehyde-3- phosphate are formed and here, first phase i.e. preparatory phase of glycolysis ends.
- In the pay-off phase, both molecules of glyceraldehyde-3-phosphate are converted to two molecules of 1, 3-bisphoglycerate by oxidation and phosphorylation.
- Here, phosphorylation is brought about with the help of inorganic phosphate and not ATP.
- Both molecules of 1, 3-bisphosphoglycerate are converted into two molecules of pyruvic acid through series of reactions accompanied with release of energy.
- This released energy is used to produce ATP (4 molecules) by substrate-level phosphorylation.
do you know ?
1. Glycolysis
- It is only source of energy production in
- erythrocytes
- renal medulla
- brain and
- sperm.
2. Some plant tissues which are modified to store starch (like potato) mainly depend upon glycolysis for energy production.
3. myoglobin of skeletal muscles is oxygen storing and transporting pigment .
- Red (dark) muscles are richer in myoglobin than the white (pale) muscles.
- Therefore, red fibers can utilize the oxygen stored in myoglobin to continue energy production over prolonged period by aerobic oxidation of glucose.
- This enables them to perform sustained work over a long period.
- On the contrary, white fibers produce the energy needed for very fast and severe work by glycolysis as sufficient oxygen is not immediately available to them for such work.
- But white muscles accumulate lactic acid and get fatigued in a short time.
- Thus athletes with a higher proportion of red fibers in their muscles are physiologically better adapted for sustained events like marathon or swimming over long distances.
Anaerobic respiration in muscle :
- In muscles, the NADH+H+ produced during glycolysis is reoxidized to NAD+ by donating one proton and two electrons to pyruvic acid which yields lactic acid.
- Skeletal muscles usually derive their energy by anaerobic respiration.
- After vigorous exercise lactic acid accumulates, leading to muscle fatigue.
- During rest, however, the lactic acid is reconverted to pyruvic acid and is channeled back into the aerobic respiration pathway.
Anaerobic respiration in yeast :
- In yeast, the pyruvate is decarboxylated to acetaldehyde.
- The acetaldehyde is then reduced by NADH+H+ to ethanol. Carbon dioxide is also produced in this process.
- This type of anaerobic respiration is termed alcoholic fermentation.
- Accumulation of ethanol by fermentation in a culture of yeast may stop further multiplication and lead to the death of cells.
- In the presence of oxygen however, yeast can respire aerobically.
Part 03 - Aerobic Respiration
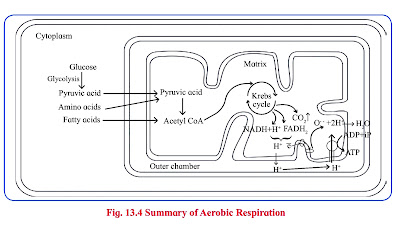
Aerobic Respiration :
- Aerobic respiration involves molecular oxygen as final electron acceptor which are liberated during oxidation of glucose.
- Glucose is completely oxidized in this process which is operated through steps like
- glycolysis
- production of acetyl CoA (connecting link reaction)
- Krebs cycle
- electron transfer chain reaction and terminal oxidation.
Glycolysis :
- First step of aerobic respiration i.e. glycolysis .
- In case of aerobic respiration, glycolytic product i.e. pyruvic acid is converted into actyl CoA.
- This process occurs in cytoplasm in case of prokaryotes and in mitochondria in case of eukaryotes.
Conversion of pyruvic acid to Acetyl CoA :
- This is an oxidative decarboxylation reaction.
- It is catalyzed by a multienzyme complex - pyruvate dehydrogenase complex (PDH).
- This enzyme is present in mitochondria of eukaryotes and cytosol of prokaryotes.This reaction is called as 'connecting link' reaction between glycolysis and Krebs cycle.
Do you know?
- Pyruvate dehydrogenase complex needs thiamin (vitamin B1) as a co-enzyme.
- It can not function in absence of vitamin B1. Hence, thiamin deficiency causes pyruvic acidosis and lactic acidosis, the life threatening conditions.
- Hence balanced diet is very important in maintenance of health.
Part 04 - Krebs Cycle
Krebs Cycle ( TCA cycle/ Citric Acid Cycle):
- Pyruvic Acid produced by glycolysis undergoes aerobic oxidation in the mitochondrial matrix through the TCA cycle.
- This cycle serves a common oxidative pathway for carbohydrates fats and proteins.
- Moreover, some intermediates of the TCA cycle are used in synthesizing important biomolecules such as glutamate and aspartate.
- Before participating in the TCA cycle pyruvic acid enters the mitochondrion.
- Here it is decarboxylated and the remaining 2-carbon fragment is combined with a molecule of coenzyme A to form acetyl-CoA.
- This reaction is an oxidative decarboxylation process and produces H+ ions and electrons along with carbon dioxide.
- During the process NAD+ is reduced to NADH+H+.
- β-oxidation of fatty acids also produces acetyl- CoA as the end product.
- Acetyl-CoA from both sources is condensed with oxaloacetic acid to form citric acid.
- Citric acid is oxidized step-wise by mitochondrial enzymes, evolving carbon dioxide. This finally regenerates oxaloacetic acid to complete the cycle.
- There are four steps of oxidation in this cycle, catalyzed by dehydrogenases (oxidoreductases) using NAD+ or FAD+ as the coenzyme.
- The coenzymes are consequently reduced to NADH+H+ and FADH2 respectively.
- These transfer their electrons to the mitochondrial respiratory chain to get reoxidised.
- One molecule of GTP (ATP) is also produced for every molecule of citric acid oxidized.
Part 05 - Amphibolic Pathway
Amphibolic Pathway :
- Aerobic respiration as catabolic (oxidative) pathway; it is not entirely correct; especially in case of Krebs cycle.
- Various reactions of Krebs cycle are mainly responsible for step-wise oxidation of acetyl part of acetyl CoA leading to release of energy and CO2.
- However, as per need, acetyl CoA or some other intermediates like α-ketoglutarate, oxaloacetate are used as precursors for synthesis of fatty acids, glutamic acid and aspartic acid respectively.
- Hence, Krebs cycle can be correctly refered to as a 'Amphibolic pathway' i.e. involving catabolism as well as anabolism.
Part 06 - Electron Transport chain
Electron Transport chain (Electron transfer system) :
- Wherever the NADH2 (NADH+H+) and FADH2 are produced during glycolysis, connecting link reaction and Krebs cycle, they are oxidised with the help of various electron carriers and enzymes.
- These carriers and enzymes are arranged on inner mitochondrial membrane in the form of various complexes as complex I, II, III, VI and V. NADH+H+ is oxidised by NADH dehydrogenase (complex I).
- It's electrons are transferred to ubiquinone (coenzyme Q CoQ) present on inner membrane of mitochondria.
- Reduced ubiquinone is called as ubiqunol.
- FADH2 is oxidised by complex II (Succinate dehydrogenase) and these electrons are also transferred to CoQ.
- During oxidation of NADH+H+ and FADH2, electrons and protons are released but only electrons are carried forward whereas protons are released into outer chamber of mitochondria.
- Ubiquinol is oxidised by complex-III (Cytochrome bc1, complex) and it's electrons are transferred to cytochrome C.
- Cytochrome C is a small, iron-containing protein, loosely associated with inner membrane.
- It acts as a mobile electron carrier, transferring the electrons between complex III and IV.
- Cytochrome C is oxidised by complex IV or cytochrome C oxidase consisting of cytochrome a and a3.
- Electrons are transferred by this complex to the molecular oxygen. This is terminal oxidation.
- Reduced molecular oxygen reacts with protons to form water molecule called as metabolic water.
- Protons necessary for this are channeled from outer chamber of mitochondria into inner chamber by F0 part of oxysome (complex V) present in inner mitochondrial membrane.
- This proton channeling by F0 is coupled to catalytic site of F1 which catalyses the synthesis of ATP from ADP and inorganic phosphate. This is oxidative phosphorylation.
- As transfer of protons is accompanied with synthesis of ATP, this process is named as 'Chemiosmosis' by Peter Mitchell.
- Oxidation of one NADH+H+ leads to production of 3 ATP molecules where as oxidation of FADH2 leads to production of 2 ATP molecules.
- However the number of ATP produced depends upon the physiological conditions and source of respiratory substrate.
Significance of ETS :
- The electron transport system (ETS) or terminal oxidation generates major amount of energy in the form of ATP molecules.
- 34 ATP molecules out of total 38 ATP molecules are produced through ETS.
- It regenerates oxidized coenzymes such as NAD+ and FAD+ from their reduced forms (NADH+H+ and FADH2) for recycling.
- It also provides water molecules necessary for Krebs cycle.
- It releases energy in a stepwise manner to prevent damage of cells.
always remember :
- Not only glucose but amino acids from protein metabolism and fatty acids from lipid metabolism also participate in Kreb's cycle through acetyl CoA.
Part 07 - Utility of stepwise oxidation
Aerobic respiration can be
demonstrated by two simple experiments.
A. A pinch of dry bakers yeast suspended in water or a few ml of yeast suspension used in a bakery is
added to about 10ml of 10 percent glucose solution in a test tube (Tube A).
B. Seed coats of a few germinating seeds (peas, beans or gram) are removed.
13.4 Utility of stepwise oxidation :
always remember
Respiratory Quotient :
Significance of Respiration
demonstrated by two simple experiments.
A. A pinch of dry bakers yeast suspended in water or a few ml of yeast suspension used in a bakery is
added to about 10ml of 10 percent glucose solution in a test tube (Tube A).
- The surface of the liquid is carefully covered with oil to prevent contact with air.
- The test tube is closed tightly with rubber stopper.
- One end of a short bent glass tube is inserted through it to reach the air inside the tube.
- Other end of the glass tube is connected by a polyethylene or rubber tubing to another bent glass tube fitted into a stopper.
- The open end of the glass tube (delivery tube) is dipped into lime water containing in a test tube (Tube B).
- Stoppers of both the tubes are fitted tightly to prevent leakage of gases.
- First test tube is placed in warm water (370C-380C) in a beaker.
- Lime water gradually turns milky, indicating the evolution of carbon dioxide from the yeast preparation.
- Level of the lime water in the delivery tube does not rise, showing that there is no decline in volume of gas in test tube A and consequently no utilization of oxygen by yeast.
- Preparation is stored for a day or two.
- When you open the stopper of tube A. You will notice a smell of alcohol indicating the formation of ethanol.
- From this activity it may be inferred that yeast respires anaerobically to ferment glucose to ethanol and carbon dioxide.
B. Seed coats of a few germinating seeds (peas, beans or gram) are removed.
- Seeds are then put in a test tube filled with mercury.
- After closing the test tube with the thumb, it is vertically inverted in a trough of mercury and the thumb is carefully removed.
- Being lighter than mercury, the seeds rise to the closed upper end of the test tube.
- No gas is seen at first in the test tube.
- As germination proceeds, a gas begins to collect at the top of the mercury in the test tube.
- On introducing a pellet of potassium hydroxide into the tube, it rises to the top and absorbs the gas.
- The mercury again fills the tube. The potassium hydroxide reacts with carbon dioxide gas to produce potassium carbonate and water.
- The gas therefore disappears.
- Evidently germinating seeds produce carbon dioxide by anaerobic respiration in the absence of oxygen in the mercury column.
13.4 Utility of stepwise oxidation :
- A stepwise release of the chemical bond energy facilitates the utilization of a relatively higher proportion of that energy in ATP synthesis.
- Activities of enzymes for the different steps may be enhanced or inhibited by specific compounds.
- This provides a means of controlling the rate of the pathway and the energy output according to need of the cell.
- The same pathway may be utilized for forming intermediates used in the synthesis of other biomolecules like amino acids.
always remember
- Removal of Hydrogen from respiratory materials is the primary process in respiration : The fact that during respiration oxygen is taken in and carbon dioxide is given out may give a false impression that respiratory materials directly unite with oxygen.
- It must be remembered that oxygen does not play such a primary role in the process of respiration.
- The primary process in respiration consists in removal of hydrogen from the respiratory materials.
- The reactions in which hydrogen is removed are catalyzed by enzymes called dehydrogenases free hydrogen cannot exists in the cell.
- As soon as it is removed from respiratory material it is picked up by substances known as acceptors.
- In aerobic respiration this hydrogen is ultimately handed over to oxygen.
- These two combine with each other and form water.
- Comparison of overall equations of photosynthesis and respiration show that to some extent, two process are reverse of each other.
- Photosynthesis involves reduction of CO2 and respiration involves oxidation of glucose.
Part 08 - Respiratory Quotient
Respiratory Quotient :
- Ratio of volume of CO2 released to the volume of O2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio.
- It depends on the type of respiratory substrate.
- When carbohydrates are used as respiratory substrate and are completely oxidized, the RQ is 1, because volume of CO2 evolved is equal to volume of O2 consumed, as shown in the equation.
- When fats or proteins are used as a substrate, the RQ is less than 1, as volume of CO2 evolved is always less than volume of O2 consumed.
- Mostly for fats, RQ is about 0.7 and for proteins it is about 0.9
- In case of anaerobic respiration RQ is always infinity as CO2 is evolved without taking O2.
Part09 - Significance of Respiration
Significance of Respiration
- Respiration provides energy for biosynthesis of cellular materials such as carbohydrates, proteins, fats, lipids, vitamins, pigments etc.
- It is also a source of energy for cell division, growth, repairs and replacement of worn out parts, movements, locomotion etc.
- Various intermediates of Krebs cycle are used as building blocks for synthesis of other complex compounds.
- Coupled with photosynthesis, it helps to maintain the balance between CO2 and O2 in the atmosphere.
- Anaerobic respiration (fermentation) is used in various industries such as diaries, bakeries, distilleries, leather industries, paper industries etc.
- It is used in the commercial production of alcohol, organic acids, vitamins, antibiotics etc.
- Energy of respiration is also used to convert insoluble substances into soluble form.
Source from Internet








No comments:
Post a Comment